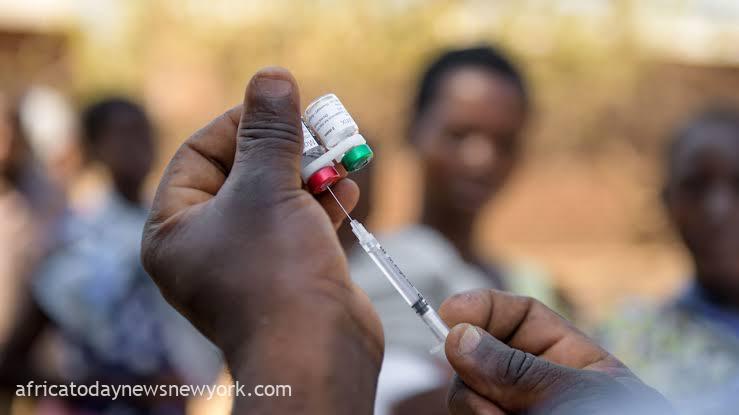
Cameroon has officially commenced the globe’s first routine vaccination effort against malaria this Monday.
According to Gavi, the global vaccine alliance, this year will see the introduction of the drug in one of the 20 African countries planning to do so.
GSK’s groundbreaking RTS,S malaria vaccine, known as Mosquirix, garnered approval from the World Health Organization in July 2022 and recently unveiled a new laboratory.
Targeting P. falciparum, the most lethal malaria strain transmitted by anopheles mosquitoes and prevalent in Africa, the vaccine is tailored for administration in four doses to young children from approximately 5 months old.
The vaccine underwent rigorous trials in Ghana, Kenya, and Malawi.
Nearly 40 years of research and development culminated in the creation of the vaccine.The vaccine’s origins, as stated by GSK, can be traced to the year 1987.
The drugmaker reported receiving funding in 2001 specifically for the development of the RTS,S-based vaccine tailored for young children.
Read also: Nigeria Vows To Defeat Malaria, Bears 30% Global Burden
In July 2023, 18 million doses of RTS,S designated for 2023–2025 were assigned to 12 countries, with a priority on regions where the risk of malaria-related illness and death among children is most severe. This prioritization continues until vaccine supply fully meets demand.
GSK’s 2015 disclosure of extensive clinical trial findings highlighted the vaccine’s relatively low effectiveness, showing a reduction in the risk of severe malaria by approximately 30 percent.
However, some researchers say it could be higher if given just ahead of the malaria season.
The fact that it must be administered across at least four doses has raised concerns about the logistics of fully inoculating children in remote areas.
The vaccine has garnered interest from over 30 African countries looking to incorporate it into their health programs.
The World Health Organization projects a demand for 40-60 million doses annually by 2026, increasing to 80-100 million by 2030.
For the next few years, demand for the vaccine is projected to outpace supply, but the expected introduction of a second vaccine is anticipated to relieve these supply constraints.
Gavi hints at a potential May or June launch for the R21 vaccine from Oxford University. A significant regulatory milestone was achieved in December with WHO pre-qualification.