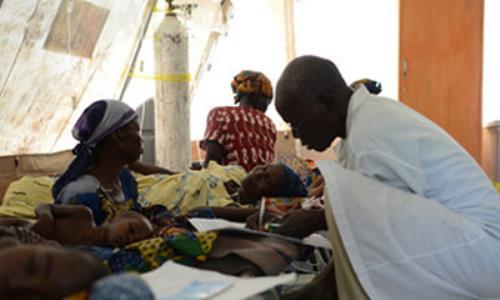
Many Children in the Chadian remote village of Gouro in northern Chad have developed paralysis following vaccinations with “MenAfriVac,” a new meningitis vaccine developed specifically for Africa by the Bill and Melinda Gates Foundation.
Fifty of the children who took the vaccine were said to have reacted to it within 24 hours after the injection was given.
According to some of the parents, the children first complained of Malaria and high temperature after which they bent over and started vomiting and then fell to the ground with convulsions.
The local doctors tried to administer a relaxant to counter the vaccine action, all to no avail. All the children ended up paralysed and vegetative after 48 hours of induced come.
Read Also: Bill Gates: Coronavirus Vaccine Will Work Well In Africa
According to a confirmed report by a cousin (whom we shall refer to as Mr. M) of two of the affected children who currently remain critically ill and hospitalised.
“Many of the children reacted within 24 hours of receiving the vaccine. At first, the children vomited and complained of headaches, before falling to the floor with uncontrollable convulsions while bent over with saliva coming from their mouths.”
“On December 26, 2012, the Ministers of Health and Social Security visited Gouro, along with two Members of Parliament to evacuate approximately 50 paralysed children to a hospital in the capital N’Djamena, over three hundred miles away.”
Government’s response to the tragedy, he added, was through paying the obviously traumatized and confused parents money in a desperate bid to silence them.
A member of the medical staff, as well as Dr. Daugla Oumagoum Moto, the director of the Center of International Health Support (CSSI), said the reactions are not consistent with this type of vaccine against meningitis, which they say are normally fever, vomiting, and headaches, not the adverse reactions experienced by the hospitalised children.
As the days turn into weeks, the paralysed children continue to lie in their hospital beds, immobile and frightened. Their parents feel confused and worried about their children’s future health. These families deserve an explanation of how this tragedy was allowed to happen and reassurance for their new future.
This project was organized and paid for by the leading organizations governing vaccines today, groups with millions of dollars available to make sure vaccines are as safe as possible. These groups include:
- CDC – Centers for Disease Control
- FDA – Food and Drug Administration
- BMGF – The Bill and Melinda Gates Foundation
- PATH – Program for Appropriate Technology in Health
- MVP – Meningitis Vaccine Project
- WHO – World Health Organization
- UNICEF – United Nations International Children’s Emergency Fund
Why did these groups ignore the advice of the India Serum Institute, which stated that their product must be stored within a specific cold temperature range? Why have none of these organizations issued a statement about what happened to these paralyzed children? Who is going to explain how the vaccines were stored and transported? What is the African government going to do to help the children and parents at the heart of this vaccination disaster? How many children must be injured before the use of the MenAfriVac vaccine is suspended?
Was this vaccine properly licensed?
In April 2012, OPTIMIZE Immunization Systems and Technologies for Tomorrow shared additional information about the MenAfriVac vaccine in a document authored by two individuals from PATH and one individual from WHO. The document, entitled MenAfriVac™ Planned for Use in a Controlled Temperature Chain, contained key evidence about the lack of appropriate licensure for this vaccine to be used outside of the cold chain:
“Unpublished data obtained from the vaccine manufacturer show that MenAfriVac has proven stable at temperatures of 40°C for limited periods of time. This indicates that the vaccine could be safely distributed outside of the 2°C to 8°C range for a specific period under controlled conditions during campaign activities. The data are currently being reviewed and Serum Institute of India plans to formally submit the request for a MenAfriVac license variation within the next few months.”
So, was a revised license ever granted for the use of MenAfriVac?
Apparently not. According to the report of a meeting which took place in October 2012 between the World Health Organization’s Immunizations, Vaccines and Biologicals group and the Immunization Practices Advisory Committee (IPAC), the vaccine would not be endorsed until 2013, at the earliest.
AFRICA TODAY NEWS, NEW YORK